Get Latest Exam Updates, Free Study materials and Tips

1. When a body A is in thermal equilibrium with a body B, and also separately with a body C, then B and C will be in thermal equilibrium with each other.
a) True
b) False
Answer: a
Explanation: Zeroth law of thermodynamics.
2. Which of the following were used as fixed points before 1954?
a) The ice point
b) The steam point
c) All of the mentioned
d) None of the mentioned
Answer: c
Explanation: Both of these were used.
3. What is the standard fixed point of thermometry?
a) The ice point
b) The steam point
c) The triple point of water
d) None of the mentioned
Answer: c
Explanation: After 1954, only one fixed point has been used.
4. All gases and vapours approach ideal gas behaviour at?
a) High pressure and high density
b) Low pressure and low density
c) High pressure and low density
d) Low pressure and high density
Answer: b
Explanation: Comes from ideal gas equation of state.
5. The value of ratio of the steam point temperature to the ice point temperature is?
a) 1.466
b) 1.266
c) 1.166
d) 1.366
Answer: d
Explanation: This value is a universal constant.
6. Celsius temperature of the triple point of water is ( in degree Celsius)?
a) -0.00
b) 0.00
c) 0.01
d) None of the mentioned
Answer: c
Explanation: Zero point of degree Celsius is shifted.
7. Which of the following is chosen as the standard thermometric substance?
a) Gas
b) Liquid
c) Solid
d) All of the mentioned
Answer: a
Explanation: Smallest variation is observed among different gas thermometers.
8. A real gas behaves as an ideal gas when?
a) Temperature approaches zero
b) Pressure approaches zero
c) Both temperature and pressure approaches zero
d) None of the mentioned
Answer: b
Explanation: It is a property of gas.
9. The temperature interval from the oxygen point to the gold point is divided into how many parts?
a) 2
b) 3
c) 4
d) 1
Answer: b
Explanation: Taken as international temperature scale.
10. Optical method is adopted for measuring temperatures higher than the gold point?
a) True
b) False
Answer: a
Explanation: Temperature is determined with the help of Planck’s law of thermal radiation.
11. The magnitude of mechanical work is the
a) product of the force and distance travelled perpendicular to the force
b) product of the force and distance travelled parallel to the force
c) sum of the force and distance travelled perpendicular to the force
d) sum of the force and distance travelled parallel to the force
Answer: b
Explanation: The work is done by a force as it acts upon a body moving in the direction of the force.
12. Work done by a system is taken to be
a) positive
b) negative
c) zero
d) varies according to situation
Answer: a
Explanation: In thermodynamics, work done by a system is take to be positive.
13. Work done on a system is taken to be
a) positive
b) negative
c) zero
d) varies according to situation
Answer: b
Explanation: In thermodynamics, work done on a system is take to be negative.
14. Work is a
a) point function
b) path function
c) depends on the state
d) none of the mentioned
Answer: b
Explanation: Amount of work done depends on the path the system follows.
15. Thermodynamic properties are
a) point function
b) path function
c) depends on the state
d) none of the mentioned
Answer: a
Explanation: For a given state there is a definite value for each property.
16. The differentials of point functions are
a) perfect differentials
b) exact differentials
c) all of the mentioned
d) none of the mentioned
Answer: c
Explanation: Change in thermodynamic property is independent of path and depends only on initial and final states of the system.
17. In the equation dV=(1/p)dW, (1/p) is known as
a) volume factor
b) pressure factor
c) differential factor
d) integration factor
Answer: d
Explanation: Used to convert inexact differential dW into exact differential dV.
18. Cyclic integral of a property is always
a) zero
b) one
c) infinite value
d) none of the mentioned
Answer: a
Explanation: The initial and final states of the system for a cyclic process are the same.
19. Constant pressure process is also known as
a) isopiestic process
b) isobaric process
c) all of the mentioned
d) none of the mentioned
Answer: c
Explanation: Isobaric and isopiestic means pressure being constant.
20. Work done in a quasi-static process
a) depends on the path followed
b) independent of the path followed
c) depends only on the initial and final states
d) none of the mentioned
Answer: a
Explanation: This is because work done is a path function.
21. Find the rate of conduction heat transfer through a 1.5 cm thick board(k = 0.16 W/m K), with a temperature difference of 20°C between the two sides.
a) 113 W/m2
b) 413 W/m2
c) 313 W/m2
d) 213 W/m2
Answer: c
Explanation: The rate of conduction heat transfer = k(∆T/∆x)
= (0.16 *20)/0.015 = 213 W/m2.
22. A window having area of 2m^2 has a surface temperature of 15°C and the air is blowing at 2°C across it with convection heat transfer coefficient of h = 125 W/m2K. Find the total heat transfer loss?
a) 3250 W
b) 2250 W
c) 4250 W
d) 5250 W
Answer: a
Explanation: Total heat transfer loss = h A ∆T
= 125*2*(15-2) = 3250 W.
23. A radiant heating lamp has a temperature of 1000 K with ε = 0.8. What should be the surface area to provide 250 W of radiation heat transfer?
a) 0.0035 m2
b) 0.0055 m2
c) 0.0075 m2
d) 0.0095 m2
Answer: b
Explanation: Radiation heat transfer = εσA(T4)
A = 250/[0.8 × 5.67 × 10-8× 10004] = 0.0055 m2.
24. A piston of mass 2 kg is lowered by 0.5 m. Find the work involved in the process.
a) 7.805 J
b) 8.805 J
c) 9.805 J
d) 10.805 J
Answer: c
Explanation: F = ma = 2 kg × 9.80665 m/s2 = 19.61 N
W = ∫ F dx = F ∫ dx = F ∆x = 19.61 N × 0.5 m = 9.805 J.
25. An escalator raises a 100 kg bucket of water 10 m in 60 seconds. Determine the amount of work done during the process.
a) 9807 J
b) 9307 J
c) 9507 J
d) 9107 J
Answer: a
Explanation: F = mg
W = ∫ F dx = F ∫ dx = F ∆x = 100 kg × 9.80665 m/s2 × 10 m
= 9807 J.
26. A hydraulic cylinder has a piston(cross sectional area 25cm2) and a fluid pressure of 2 MPa. If piston moves by 0.25m, how much work is done?
a) 0.25 kJ
b) 1.25 kJ
c) 2.25 kJ
d) 3.25 kJ
Answer: b
Explanation: W = ∫ F dx = ∫ PA dx = PA ∆x
= 2000 kPa × 25 × 10(-4) m2 × 0.25 m = 1.25 kJ.
27. In a thermally insulated kitchen, a refrigerator with 2 kW motor for running the compressor provides 6000 kJ of cooling to the refrigerated space during 30 min operation. If the condenser coil placed behind the refrigerator rejects 8000 kJ of heat to the kitchen during the same period, calculate the change in internal energy of the kitchen.
a) 3600 kJ
b) 2400 kJ
c) 4800 kJ
d) none of the mentioned
Answer: a
Explanation: QKitchen = 0 (Insulated!),
W(Electrical) = – P*∆τ = – 2 kW*30*60 sec = – 3600 kJ
(It is negative because work is done on to the system)
Change in internal energy of the kitchen (∆UKitchen) = QKitchen – WElectrical
= 0 – (–3600) = 3600 kJ.
28. An escalator raises a 100 kg bucket of sand 10 m in a minute. Determine the rate of work done during the process.
a) 143 W
b) 153 W
c) 163 W
d) 173 W
Answer: c
Explanation: The work is force with a displacement and force is F = mg, which is constant
W = ∫ F dx = F ∫ dx = F ∆x = 100 kg × 9.80665 m/s2 × 10 m = 9807 J
The rate of work is work per unit time = W/∆t = 9807 J / 60 s
= 163 W.
29. A crane lifts a bucket of cement with a mass of 450 kg vertically up with a constant velocity of 2 m/s. Find the rate of work.
a) 8.83 kW
b) 8.33 kW
c) 8.53 kW
d) 8.63 kW
Answer: a
Explanation: Rate of doing work = FV = mg × V = 450 kg × 9.807 ms^(−2) × 2 ms^(−1)
= 8826 J/s = 8.83 kW.
30. A battery is well insulated while being charged by 12.3 V at a current of 6 A. Take the battery as a control mass and find the instantaneous rate of work.
a) 63.8 W
b) 73.8 W
c) 83.8 W
d) 93.8 W
Answer: b
Explanation: Battery thermally insulated ⇒ Q = 0
For constant voltage E and current i, Power = E i = 12.3 × 6 = 73.8 W.
31. A current of 10 amp runs through a resistor with resistance of 15 ohms. Find the rate of work that heats the resistor up.
a) 1200 W
b) 1300 W
c) 1400 W
d) 1500 W
Answer: d
Explanation: Power = E i = R i^(2) = 15 × 10 × 10 = 1500 W.
32. A pressure of 650 kPa pushes a piston of radius 0.125 m with V = 5 m/s. What is the transmitted power?
a) 139.5 kW
b) 149.5 kW
c) 159.5 kW
d) 169.5 kW
Answer: c
Explanation: A = π/4(D)2 = 0.049087 m2;
Volume flow rate = AV = 0049087 m^2 × 5 m/s = 0.2454 m3/s
Power = F V = P(Volume flow rate) = 650 kPa × 0.2454 m3/s = 159.5 kW.
33. Air at a constant pressure in a piston-cylinder is at 300 K, 300 kPa and V=0.1 m^3. It is heated to 600 K in 30 seconds in a process with constant piston velocity. Find the power delivered to the piston.
a) 1 kW
b) 2 kW
c) 3 kW
d) 4 kW
Answer: a
Explanation: Process: P = constant : dW = P dV
V2 = V1× (T2/T1) = 0.1 × (600/300) = 0.2
Rate = P (∆V / ∆t) = 300 × (0.2-0.1)/30 = 1 kW.
34. A torque of 650 Nm rotates a shaft of radius 0.125 m with ω = 50 rad/s. What is the transmitted power?
a) 22.5 kW
b) 32.5 kW
c) 42.5 kW
d) 52.5 kW
Answer: b
Explanation: V = ωr = 50 × 0.125 = 6.25 m/s
Power = Tω = 650 × 50 Nm/s = 32 500 W = 32.5 kW.
1. Energy has different forms which include
a) heat
b) work
c) all of the mentioned
d) none of the mentioned
Answer: c
Explanation: Basic fact about energy.
2. Work input is directly proportional to heat and the constant of proportionality is called
a) joule’s equivalent
b) mechanical equivalent of heat
c) all of the mentioned
d) none of the mentioned
Answer: c
Explanation: True for a closed system undergoing a cycle.
3. The value of constant of proportionality, J, has the value
a) 1
b) 0
c) -1
d) infinity
Answer: a
Explanation: In the S.I. system, both heat and work are measured in the derived unit of energy, the Joule.
4. It was Joule who first established that heat is a form of energy, and thus laid the foundation of the first law of thermodynamics.
a) true
b) false
Answer: a
Explanation: Prior to Joule, heat was considered to be an invisible fluid flowing from a body of higher calorie to a body of lower calorie.
5. Which of the following represents the energy in storage?
a) heat
b) work
c) internal energy
d) none of the mentioned
Answer: c
Explanation: Energy in storage is internal energy or the energy of the system.
6. By first law of thermodynamics,
a) Q=ΔE-W
b) Q=ΔE+W
c) Q=-ΔE-W
d) Q=-ΔE+W
Answer: b
Explanation: Q-W is the net energy stored in system and is called internal energy of system.
7. The expression (ΣW)cycle=(ΣQ)cycle applies only to systems undergoing cycles.
a) true
b) false
Answer: a
Explanation: The above expression holds for a closed cycle.
8. Which of the following is the first law for a closed system undergoing a cycle?
a) ∫dW=∫dQ
b) J∫dW=∫dQ
c) ∫dW=J∫dQ
d) none of the mentioned
Answer: c
Explanation: This is the expression for first law of thermodynamics where ∫ denotes the cyclic integral for the closed path.
9. Which of the following an be considered as the definition of energy?
a) Q=ΔE+W
b) Q-W=ΔE
c) first law of thermodynamics
d) all of the mentioned
Answer: d
Explanation: The first law is a particular formulation of the principle of the conservation of energy.
10. The first law of thermodynamics gives only the change on energy ΔE for the process.
a) true
b) false
Answer: a
Explanation: An absolute value of energy E, is not given by the first law.
11. Macroscopic properties p and V are significant only for
a) equilibrium states
b) non-equilibrium states
c) depends on the state
d) none of the mentioned
Answer: a
Explanation: This is true for at any intermediate point in the travel of piston.
12. In a cylinder, infinitesimal amount of work done by the gas on piston is given by
a) F*dl
b) p*a*dl
c) p*dV
d) all of the mentioned
Answer: d
Explanation: F=p*a and work equals force multiplied by displacement.
13. For a constant pressure process, work done is
a) zero
b) p*(V2-V1)
c) p1*V1*ln(V2/V1)
d) none of the mentioned
Answer: b
Explanation: Work done in a process is given by the area under p-dV graph.
14. For a constant volume process, work done is
a) zero
b) p*(V2-V1)
c) p1*V1*ln(V2/V1)
d) none of the mentioned
Answer: a
Explanation: Work done in a process is zero since volume remains constant.
15. For a process in which pV=C, work done is
a) zero
b) p*(V2-V1)
c) p1*V1*ln(V2/V1)
d) none of the mentioned
Answer: c
Explanation: Work done is given by integral of (p*dV) from V1 to V2.
16. The enthalpy of a substance(denoted by h), is defined as
a) h=u-pv
b) h=u+pv
c) h=-u+pv
d) h=-u-pv
Answer: b
Explanation: This is a basic definition for enthalpy.
17. In a constant volume process, internal energy change is equal to
a) heat transferred
b) work done
c) zero
d) none of the mentioned
Answer: a
Explanation: In a constant volume process, there is no work other than the pdV work.
18. For an ideal gas, enthalpy becomes
a) h=u-RT
b) h=-u-RT
c) h=u+RT
d) h=-u+RT
Answer: c
Explanation: For an ideal gas, pv=RT.
19. Enthalpy is an intensive property of a system.
a) true
b) false
Answer: a
Explanation: Enthalpy is an intensive property measured mostly in kJ/kg.
20. Heat transferred at constant pressure _____ the enthalpy of a system.
a) decreases
b) increases
c) first decreases then increases
d) first increases then decreases
Answer: b
Explanation: At constant pressure, (dQ)=dh where h=u+pv is the specific enthalpy of the system.
21. The enthalpy of an ideal gas depends only on the temperature.
a) true
b) false
Answer: a
Explanation: This is because the internal energy of an ideal gas depends only on the temperature.
21. The specific heat of a substance at constant volume is defined as the rate of change of ___ with respect to ___
a) specific internal energy, temperature
b) work, pressure
c) specific internal energy, pressure
d) heat, temperature
Answer: a
Explanation: cv=∂u/∂T at constant volume.
22. Heat transferred at constant _____ increases the _____ of a system.
a) pressure, increases
b) volume, increases
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: At constant pressure, (dQ)=dh and at constant volume, Q=Δu.
23. Specific heat of a substance at constant volume is a property of the system.
a) true
b) false
Answer: a
Explanation: Since T,v and u are the properties of the system, specific heat at a constant volume is a property of the system.
24. The specific heat of a substance at constant pressure is defined as the rate of change of ___ with respect to ___
a) work, pressure
b) enthalpy, temperature
c) enthalpy, pressure
d) heat, temperature
Answer: b
Explanation: cp=∂h/∂T at constant pressure.
25. The heat capacity at constant pressure Cp
a) m/cp
b) cp/m
c) mcp
d) none of the mentioned
Answer: c
Explanation: Cp=(mass*specific heat at constant pressure).
26. Specific heat of a substance at constant pressure is a property of the system.
a) true
b) false
Answer: a
Explanation: cp is a property of a substance just like cv.
27. Equation of continuity comes from
a) conservation of energy
b) conservation of mass
c) conservation of work
d) conservation of heat
Answer: b
Explanation: w1=w2 i.e., we get (AV/v)1=(AV/v)2 and this is called equation of continuity(where w1 & w2 are mass flow rates).
28. In a flow process, the work transfer may be of which type?
a) external work
b) flow work
c) all of the mentioned
d) none of the mentioned
Answer: c
Explanation: Flow work is the displacement work and external work mostly comprises of shaft work.
29. The total rate of flow of all energy streams entering the control volume must equal to that of leaving the control volume.
a) true
b) false
Answer: a
Explanation: Given statement is true by the conservation of energy.
30. Which of the following represents the steady flow energy equation?
a) Q+Wx=(h2-h1)-(V2-V1)(V2+V1)/2+g(Z2-Z1)
b) Q+Wx=(h2-h1)+(V2-V1)(V2+V1)/2+g(Z2-Z1)
c) Q-Wx=(h2-h1)-(V2-V1)(V2+V1)/2+g(Z2-Z1)
d) Q-Wx=(h2-h1)+(V2-V1)(V2+V1)/2+g(Z2-Z1)
Answer: d
Explanation: This equation is the general form of SFEE and it involves conservation of mass and energy.
31. When more than one fluid stream is in a control volume, which of the following is more convenient?
a) energy flow per unit time
b) energy flow per unit mass
c) all of the mentioned
d) none of the mentioned
Answer: a
Explanation: It makes calculations less difficult.
32. In the differential form, the SFEE becomes
a) dQ+dW=dh+VdV+gdZ
b) dQ-dW=dh+VdV+gdZ
c) dQ+dW=dh-VdV-gdZ
d) dQ-dW=dh-VdV+gdZ
Answer: b
Explanation: This equation is the differential form of SFEE.
33. The steady flow energy equation is applied to which of the following processes?
a) pipe line flows
b) heat transfer processes
c) combustion processes
d) all of the mentioned
Answer: d
Explanation: These are the applications of SFEE.
34. When more than one fluid stream enters or leaves the control volume, which type of balance is taken?
a) mass balance
b) energy balance
c) mass balance and energy balance
d) none of the mentioned
Answer: c
Explanation: Both energy and mass balance are considered here.
35. What are the different kinds of external work?
a) shear work
b) electrical work
c) all of the mentioned
d) none of the mentioned
Answer: c
Explanation: Given two kinds of external work are important.
36. The flow work is the displacement work done by the fluid and is given by
a) -pvdm
b) pvdm
c) pvdm or -pvdm depending on whether it is inlet or exit
d) none of the mentioned
Answer: c
Explanation: At inlet, flow work=-pvdm and at exit, flow work=pvdm.
37. What does a nozzle do?
a) decreases the velocity of a fluid at the cost of its pressure gain
b) increases the velocity of a fluid at the cost of its pressure drop
c) increases the velocity of a fluid and also its pressure
d) none of the mentioned.
Answer: b
Explanation: A nozzle increases KE of fluid and reduces its pressure.
38. What does a diffuser do?
a) increases the pressure of the fluid at the expense of its KE
b) decreases the pressure of the fluid and also increases its KE
c) increases the pressure of the fluid and also its KE
d) decreases the pressure of the fluid and also its KE
Answer: a
Explanation: A diffuser increases the pressure at the expense of its KE.
39. For an insulated nozzle, SFEE of the control surface gives ( considering change in PE is zero and inlet velocity is small compared to exit velocity)
a) V2=sqrt(4*Δh)
b) V2=sqrt(Δh)
c) V2=sqrt(Δh/2)
d) V2=sqrt(2*Δh)
Answer: d
Explanation: dQ/dm=0, dW/dm=0, Δh=h1-h2.
40. Fluid flow through which of the following throttles the flow?
a) partially opened valve
b) orifice
c) porous plug
d) all of the mentioned
Answer: d
Explanation: In all of the given cases, there is an appreciable drop in pressure and hence the flow is throttled.
41. In a throttling device, what do we get as SFEE when changes in PE and KE are taken zero?
a) dQ/dm≠0
b) dW/dm≠0
c) h1=h2
d) none of the mentioned
Answer: c
Explanation: Enthalpy of the fluid before throttling is equal to the enthalpy of the fluid after throttling.
42. Turbines and engines ____ positive power output, and compressors and pumps ____ power input.
a) require, give
b) give, require
c) give, give
d) require, require
Answer: b
Explanation: This is the basic information about turbines, engines, compressors and pumps.
43. For a turbine, it is seen that work is done by the fluid at the expense of its enthalpy.
a) true
b) false
Answer: a
Explanation: For a turbine, W/m=h1-h2.
44. For an adiabatic compressor or pump,
a) the enthalpy of fluid remains constant with the amount of work input
b) the enthalpy of fluid decreases by the amount of work input
c) the enthalpy of fluid increases by the amount of work input
d) none of the mentioned
Answer: c
Explanation: For an adiabatic pump or compressor, W/m=h2-h1.
45. A heat exchanger is a device in which heat is transferred from one fluid to another.
a) true
b) false
Answer: a
Explanation: Basic fact about heat exchanger.
46. For an inviscid frictionless fluid flowing through a pipe, Euler equation is given by
a) Vdp+VdV+gdZ=0
b) Vdp-VdV+gdZ=0
c) Vdp-VdV-gdZ=0
d) none of the mentioned
Answer: a
Explanation: Euler equation is derived from steady flow energy equation.
47. The Bernoulli equation is restricted to _____ fluids but the SFEE is valid for _____ fluids as well.
a) viscous compressible, frictionless incompressible
b) frictionless incompressible, viscous compressible
c) viscous incompressible, frictionless compressible
d) none of the mentioned
Answer: b
Explanation: This statement tells us that the Bernoulli equation is a limiting case of SFEE.
2. According to Joule’s experiments,
a) heat can be completely converted into work
b) work can be completely converted into heat
c) both heat and work are completely interchangeable
d) all of the mentioned
Answer: b
Explanation: Work transfer -> internal energy increase -> heat transfer.
3. Which of the following is true?
a) work is a high grade energy
b) heat is a low grade energy
c) complete conversion of low grade energy into high grade energy in a cycle is impossible
d) all of the mentioned
Answer: d
Explanation: These facts are in accordance with Joule’s work and underlies the work of Carnot.
4. In a cyclic heat engine there is
a) net heat transfer to the system and net work transfer from the system
b) net heat transfer from the system and net work transfer to the system
c) depends on the conditions of cycle
d) none of the mentioned
Answer: a
Explanation: This is the basic concept of cycle heat engine.
5. Boiler, turbine, condenser and pump together constitute a heat engine.
a) true
b) false
Answer: a
Explanation: It is an example for a cyclic heat engine.
6. In a heat engine cycle, which of the following process occurs?
a) heat is transferred from furnace to boiler
b) work is produced in turbine rotor
c) steam is condensed in condenser
d) all of the mentioned
Answer: d
Explanation: These are the basic processes occurring in a heat engine cycle comprising of furnace, boiler condenser and a turbine.
7. The function of a heat engine cycle is to _____ continuously at the expense of _____ to the system.
a) heat input, produce work
b) produce work, heat input
c) can be both of the mentioned
d) none of the mentioned
Answer: b
Explanation: Net work and heat input are of primary interest in a cycle.
8. Efficiency of a heat engine is defined as
a) total heat output / net work input
b) total heat input / net work output
c) net work output / total heat input
d) net work input / total heat output
Answer: c
Explanation: Basic definition of efficiency.
9. A thermal energy reservoir is a large body of
a) small heat capacity
b) large heat capacity
c) infinite heat capacity
d) none of the mentioned
Answer: c
Explanation: Basic fact about TER.
10. Processes inside a thermal energy reservoir are quasi-static.
a) true
b) false
Answer: a
Explanation: The changes taking place in TER are very slow and minute.
11. Which device maintains a body at a temperature lower than the temperature of the surroundings?
a) PMM1
b) PMM2
c) refrigerator
d) heat pump
Answer: c
Explanation: This is the main function of a refrigerator.
12. What does a refrigerant do?
a) absorbs the heat leakage into body from surroundings
b) evaporates in the evaporator
c) absorbs latent heat of vaporization form the body which is cooled
d) all of the mentioned
Answer: d
Explanation: Refrigerant is required for the proper functioning of a refrigerator.
13. Coefficient of performance(COP) is defined as
a) heat leakage/work input
b) work input/heat leakage
c) latent heat of condensation/work input
d) work input/latent heat of condensation
Answer: a
Explanation: Coefficient of performance is the performance parameter used in a refrigerator cycle.
14. Which device maintains a body at a temperature higher than the temperature of the surroundings?
a) PMM1
b) PMM2
c) refrigerator
d) heat pump
Answer: d
Explanation: This is the main function of a heat pump.
15. In a heat pump, there is heat leakage from the body to the surroundings.
a) true
b) false
Answer: a
Explanation: This is just opposite to a refrigerator.
16. What is the relation between COP of heat pump and refrigerator?
a) COP of pump=COP of refrigerator – 1
b) COP of pump=COP of refrigerator + 1
c) COP of pump=COP of refrigerator – 2
d) COP of pump=COP of refrigerator + 2
Answer: b
Explanation: This relation comes from the COP of pump and refrigerator.
17. Which device maintains a body at a temperature lower than the temperature of the surroundings?
a) PMM1
b) PMM2
c) refrigerator
d) heat pump
Answer: c
Explanation: This is the main function of a refrigerator.
18. What does a refrigerant do?
a) absorbs the heat leakage into body from surroundings
b) evaporates in the evaporator
c) absorbs latent heat of vaporization form the body which is cooled
d) all of the mentioned
Answer: d
Explanation: Refrigerant is required for the proper functioning of a refrigerator.
19. Coefficient of performance(COP) is defined as
a) heat leakage/work input
b) work input/heat leakage
c) latent heat of condensation/work input
d) work input/latent heat of condensation
Answer: a
Explanation: Coefficient of performance is the performance parameter used in a refrigerator cycle.
20. Which device maintains a body at a temperature higher than the temperature of the surroundings?
a) PMM1
b) PMM2
c) refrigerator
d) heat pump
Answer: d
Explanation: This is the main function of a heat pump.
21. In a heat pump, there is heat leakage from the body to the surroundings.
a) true
b) false
Answer: a
Explanation: This is just opposite to a refrigerator.
22. Carnot cycle is a reversible cycle.
a) true
b) false
Answer: a
Explanation: A reversible cycle is an ideal hypothetical cycle in which all processes are reversible.
23. A reversible cycle has following processes.
a) 4 isothermal processes
b) 4 adiabatic processes
c) 2 isothermal and 2 adiabatic processes
d) none of the mentioned
Answer: c
Explanation: Two reversible isotherms and two reversible adiabatics constitute a Carnot cycle.
24. The correct sequence of the processes taking place in a carnot cycle is
a) adiabatic -> adiabatic -> isothermal -> isothermal
b) adiabatic -> isothermal -> adiabatic -> isothermal
c) isothermal -> isothermal -> adiabatic -> adiabatic
d) isothermal -> adiabatic -> isothermal -> adiabatic
Answer: d
Explanation: Carnot cycle consists if these four processes in succession.
25. The reversed heat engine takes heat from a ___ temperature body, then discharges it to a ___ temperature body and ___ an inward flow of network.
a) high, low, receives
b) low, high, receives
c) high, low, gives
d) low, high, gives
Answer: b
Explanation: In reversed heat engine, the magnitude of energy transfers remains same and only directions change.
26. Example of reversed heat engine is
a) heat pump
b) refrigerator
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: Heat pump and refrigerator are the types of reversed heat engine.
27. Integral of dQ/T is independent of reversible path connecting between two points.
a) true
b) false
Answer: a
Explanation: For two reversible paths, dQ/T doesn’t depend on the path taken.
28. Integral of dQ/T of a reversible path is given by
a) Si-Sf
b) Sf-Si
c) Si+Sf
d) -Si-Sf
Answer: b
Explanation: Integral of dQ/T is = Sf-Si where i=initial equilibrium state and f=final equilibrium state.
29. Entropy is a
a) path function, intensive property
b) path function, extensive property
c) point function, intensive property
d) point function, extensive property
Answer: d
Explanation: Fact about entropy and unit of entropy is J/K.
30. Specific entropy is given by( where m is the mass)
a) Sm
b) m/S
c) S/m
d) none of the mentioned
Answer: c
Explanation: s=S/m with unit J/kg K.
31. For any process which is undergone by a system
a) dQ/T>=ds
b) dQ/T<=ds
c) dQ/T≠ds
d) none of the mentioned
Answer: b
Explanation: For any process dQ/T<=ds and this comes from Clausius theorem.
32. For a reversible heat transfer and process being adiabatic, which of the following is true?
a) dQ=0
b) dS=0
c) S=constant
d) all of the mentioned
Answer: d
Explanation: dQ=0 since process is reversible and adiabatic and dS=dQ/T.
33. A reversible adiabatic process is an isentropic process.
a) true
b) false
Answer: a
Explanation: dQ=0 and dS=0 and hence S=constant.
34. The area under the curve ∫TdS is equal to the
a) work done
b) heat transferred
c) internal energy change
d) none of the mentioned
Answer: b
Explanation: Q(reversible)=∫TdS.
35. Which of the following statement is true?
a) for reversible isothermal heat transfer, Q=t(Sf-Si)
b) for reversible adiabatic process, S=constant
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: For reversible isothermal heat transfer, T=constant and for reversible adiabatic process, dS=0.
36. A Carnot cycle has following processes.
a) 4 reversible isotherms
b) 4 reversible adiabatics
c) 2 reversible isotherms and 2 reversible adiabatics
d) none of the mentioned
Answer: c
Explanation: Two reversible isotherms and two reversible adiabatics constitute a Carnot cycle.
37. Net work in a Carnot cycle is given by (T1=temperature of heat addition and T2=temperature of heat rejection)
a) (T2-T1)(S1-S4)
b) (T1-T2)(S1-S4)
c) (T1-T2)(S4-S1)
d) none of the mentioned
Answer: b
Explanation: Net work=Q1-Q2=(T1-T2)(S1-S4).
38. According to the principle of Caratheodory, the first law in differential form is written as dQ=Adx+Bdy+Cdz.
a) true
b) false
Answer: a
Explanation: Here, x,y,z are the three thermodynamic coordinates and A,B,C are the functions of x,y,z.
39. For adiabatic, reversible transition,
a) Adx+Bdy+Cdz=-1
b) Adx+Bdy+Cdz=1
c) Adx+Bdy+Cdz=0
d) none of the mentioned
Answer: c
Explanation: dQ=Adx+Bdy+Cdz=0 for adiabatic and reversible process.
40. For quasi-static, adiabatic path
a) Adx+Bdy+Cdz=TdS
b) Adx+Bdy+Cdz=1
c) Adx+Bdy+Cdz=0
d) none of the mentioned
Answer: a
Explanation: This comes from Caratheodory’s theorem.
41. The infinitesimal change in entropy dS due to reversible heat transfer dQ at temperature T is dS=dQ/T.
a) true
b) false
Answer: a
Explanation: For a reversible process, dS=dQ/T .
42. A heat pump is used to meet the heating requirements of a house and maintain it at 20°C. On a day when the outdoor air temperature drops to 2°C, the house is estimated to lose heat at a rate of 80,000
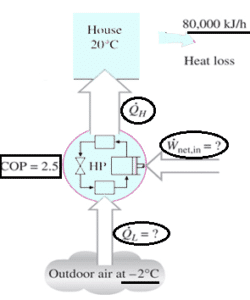
kJ/h. If the heat pump under these conditions has a COP of 2.5, determine the power consumed by the heat pump.
a) 32000 kJ/h
b) 33000 kJ/h
c) 34000 kJ/h
d) 35000 kJ/h
Answer: a
Explanation: W = Q/COP = 80000 kJ/h / 2.5 = 32000 kJ/h.
43. A heat pump is used to meet the heating requirements of a house and maintain it at 20°C. On a day when the outdoor air temperature drops to 2°C, the house is estimated to lose heat at a rate of 80,000
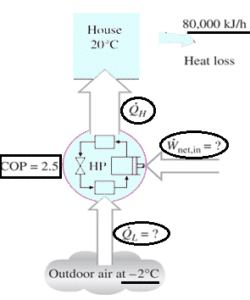
kJ/h. If the heat pump under these conditions has a COP of 2.5, determine the rate at which heat is absorbed from the cold outdoor air.
a) 32000 kJ/h
b) 48000 kJ/h
c) 54000 kJ/h
d) 72000 kJ/h
Answer: b
Explanation: The rate at which heat is absorbed = 80000 – 32000 = 48000 kJ/h.
44. An air-conditioner provides 1 kg/s of air at 15°C cooled from outside atmospheric air at 35°C. Estimate the amount of power needed to operate the air-conditioner.
a) 1.09 kW
b) 1.19 kW
c) 1.29 kW
d) 1.39 kW
Answer: d
Explanation: Q = m*cp*(temperature change) = 20.08 kW
COP = (15+273)/(35-15) = 14.4
hence power needed = 20/14.4 = 1.39 kW.
45. A cyclic machine, as shown below, receives 325 kJ from a 1000 K energy reservoir. It rejects 125 kJ to a 400 K energy reservoir and the cycle produces 200kJ of work as output. Is this cycle reversible, irreversible, or impossible?
a) reversible
b) irreversible
c) impossible
d) none of the mentioned
Answer: c
Explanation: The Carnot efficiency = 1 – (400/1000) = 0.6 and real efficiency = (300/325) = 0.615 which is greater than the Carnot efficiency hence cycle is impossible.
46. In a cryogenic experiment you need to keep a container at -125°C although it gains 100 W due to heat transfer. What is the smallest motor you would need for a heat pump absorbing heat from the container and rejecting heat to the room at 20°C?
a) 97.84 kW
b) 98.84 kW
c) 99.84 kW
d) 95.84 kW
Answer: a
Explanation: COP = 1.022 and thus power required = 100/1.022 = 97.84 kW.
1. Which of the following is a type of energy?
a) high grade energy
b) low grade energy
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: These are two types in which the sources of energy can be divided into.
2. Which of the following is an example of high grade energy?
a) mechanical work
b) electrical energy
c) water power and wind power
d) all of the mentioned
Answer: d
Explanation: These are some examples of the high grade energy.
3. The complete conversion of heat into shaft-work is impossible.
a) true
b) false
Answer: a
Explanation: This statement can be proved by the second law of thermodynamics.
4. Which of the following is an example of low grade energy?
a) heat or thermal energy
b) heat from nuclear fission or fusion
c) heat from combustion of fossil fuel
d) all of the mentioned
Answer: d
Explanation: These are few examples of low grade energy.
5. The part of ____ available for conversion is referred to ____
a) high grade energy, available energy
b) low grade energy, available energy
c) low grade energy, unavailable energy
d) high grade energy, unavailable energy
Answer: b
Explanation: Only some part of low grade energy is available for conversion.
6. The ____ obtainable from a certain heat input in a cyclic heat engine is called ____
a) minimum work output, available energy
b) maximum work output, available energy
c) minimum work input, unavailable energy
d) none of the mentioned
Answer: b
Explanation: Q1=AE+UE and the minimum energy that has to be rejected is called the unavailable energy.
7. The unavailable energy is the product of the lowest temperature of heat rejection and the change of entropy of system during the process of supplying heat.
a) true
b) false
Answer: a
Explanation: U.E.=T0*(change in entropy).
8. The lowest practicable temperature of heat rejected is the
a) given temperature
b) 0K
c) temperature of surroundings
d) 273K
Answer: c
Explanation: Work done and hence efficiency will be maximum when heat is rejected at the temperature of surroundings.
9. The available energy is known as ____ and the unavailable energy is known as ____
a) energy, exergy
b) exergy, energy
c) both are called exergy
d) both are called energy
Answer: b
Explanation: Rant was the one who coined these terms.
10. Whenever heat is transferred through a finite temperature difference, there is always a decrease in the availability of energy so transferred.
a) true
b) false
Answer: a
Explanation: This is because of exergy lost due to irreversible heat transfer.
11. For a process from state 1 to state 2, heat transfer in a reversible process is given by
a) Q for reversible=(To)*(S1-S2)
b) Q for reversible=(To)*(S2-S1)
c) Q for reversible=(To)/(S1-S2)
d) Q for reversible=(To)/(S2-S1)
Answer: b
Explanation: To is the temperature of the surroundings and S1,S2 are the entropies at state 1 and 2 respectively and ΔS(universe)=0.
12. For a process from state 1 to state 2, heat transfer in an irreversible process is given by
a) Q for irreversible=(To)*(S1-S2)
b) Q for irreversible>(To)*(S1-S2)
c) Q for irreversible<(To)*(S1-S2)
d) none of the mentioned
Answer: c
Explanation: To is the temperature of the surroundings and S1,S2 are the entropies at state 1 and 2 respectively ans ΔS(universe)>0.
13. Which of the following is true?
a) Q for reversible > Q for irreversible and work for reversible < work for irreversible
b) Q for reversible < Q for irreversible and work for reversible > work for irreversible
c) Q for reversible < Q for irreversible and work for reversible < work for irreversible
d) Q for reversible > Q for irreversible and work for reversible > work for irreversible
Answer: d
Explanation: This is because, Q for reversible=(To)*(S2-S1) and Q for irreversible<(To)*(S1-S2).
14. Work done in all reversible processes is equal.
a) true
b) false
Answer: a
Explanation: Reversible processes between the same end states must coincide and and produce equal amounts of work.
15. In an open system, for maximum work, the process must be entirely
a) irreversible
b) reversible
c) adiabatic
d) none of the mentioned
Answer: b
Explanation: A reversible process gives the maximum work.
16. Which of the following is true for a steady flow system?
a) mass entering = mass leaving
b) mass does not enter or leave the system
c) mass entering can be more or less than the mass leaving
d) none of the mentioned
Answer: a
Explanation: For a steady flow process, mass entering the system is equal to the mass leaving the system.
17. When we obtain useful work during a process in which a finite system undergoes a change of state, when should that process terminate?
a) when the pressure of system equals the pressure of surroundings
b) when the temperature of system equals the temperature of surroundings
c) when the system has reached the dead state
d) all of the mentioned
Answer: d
Explanation: The process goes on until the system reaches the dead state.
18. The availability(A) of a given system is defined as the ____ work that is obtainable in a process in which system comes to equilibrium with its surroundings.
a) useful work
b) maximum useful work
c) minimum useful work
d) none of the mentioned
Answer: b
Explanation: Maximum useful work is given by total work minus pdV work.
19. Availability is a composite property.
a) true
b) false
Answer: a
Explanation: This is because it depends on the state of both the system and surroundings.
20. Availability function for a steady flow system is given by
a) H+TS+(m*V*V/2)+(m*g*z)
b) H-TS+(m*V*V/2)+(m*g*z)
c) H-TS-(m*V*V/2)-(m*g*z)
d) H-TS-(m*V*V/2)+(m*g*z)
Answer: b
Explanation: This term comes very frequent and is considered as availability function for a steady flow system.
21. Availability function for a closed system is given by
a) u-pv-Ts
b) u+pv+Ts
c) u-pv+Ts
d) u+pv-Ts
Answer: d
Explanation: This term comes very frequent and is considered as availability function for a closed system.
22. The compressor in a refrigerator takes R-134a in at 100 kPa, −20°C and then compresses it to 1 MPa, 40°C. With the room temperature at 20°C find the minimum compressor work.
a) -48.19 kJ/kg
b) -58.19 kJ/kg
c) -68.19 kJ/kg
d) -78.19 kJ/kg
Answer: a
Explanation: w(c) = h1 – h2 + q(rev)
w(min) = h1 – h2 + To(s2 – s1) = 387.22 – 420.25 + 293.15 × (1.7148 – 1.7665)
= -48.19 kJ/kg.
23. Find the specific reversible work for a steam turbine with inlet at 4 MPa, 500°C and an actual exit state of 100 kPa, x = 1.0 with a 25°C ambient temperature.
a) 550.0 kJ/kg
b) 650.0 kJ/kg
c) 750.0 kJ/kg
d) 850.0 kJ/kg
Answer: d
Explanation: To = 25°C = 298.15 K, hi = 3445.2 kJ/kg; si = 7.090 kJ/kg K,
he = 2675.5 kJ/kg; se = 7.3593 kJ/kg K
w(rev) = (hi – Tosi) – (he – Tose) = (hi – he) + To(se – si)
= (3445.2 – 2675.5) + 298.2(7.3593 – 7.0900)
= 769.7 + 80.3 = 850.0 kJ/kg.
24. Find the specific reversible work for a compressor using R-134a with inlet state of –20°C, 100 kPa and an exit state of 50°C, 600 kPa. Use 25°C as ambient temperature.
a) -28.878 kJ/kg
b) -38.878 kJ/kg
c) -48.878 kJ/kg
d) -58.878 kJ/kg
Answer: b
Explanation: The compressor is assumed to be adiabatic so q = 0
w(rev) = To(se – si) – (he – hi)
hi = 387.22 kJ/kg; si = 1.7665 kJ/kg K;
he = 438.59 kJ/kg; se = 1.8084 kJ/kg K
w(rev) = 298.15 (1.8084 – 1.7665) – (438.59 – 387.22)
= -38.878 kJ/kg.
25. A steady stream of R-22 at ambient temperature of 10°C, and at 750 kPa enters a solar collector. The stream exits at 80°C, 700 kPa. Calculate the change in availability.
a) 4.237 kJ/kg
b) 5.237 kJ/kg
c) 6.237 kJ/kg
d) 7.237 kJ/kg
Answer: c
Explanation: hi = 56.46 kJ/kg, si = 0.2173 kJ/kg K,
he = 305.91 kJ/kg, se = 1.0761 kJ/kg K
∆ψie = ψe – ψi = (he – hi) – T0(se – si)
= (305.912 – 56.463) – 283.2(1.0761 – 0.2173)
= 6.237 kJ/kg.
26. Cold water is running in a river at 2°C and the air temperature is 20°C. What is the availability of water relative to the ambient temperature?
a) 2.157 kJ/kg
b) 2.857 kJ/kg
c) 3.457 kJ/kg
d) 2.457 kJ/kg
Answer: d
Explanation: ψ = h1 – h0 – T0(s1 – s0)
ψ = 8.392 – 83.96 – 293.15(0.03044 – 0.2966)
= 2.457 kJ/kg.
27. Which of the following represents the specific volume during phase transition.
a) Vf-Vg
b) Vg-Vf
c) Vf+Vg
d) none of the mentioned
Answer: b
Explanation: Here Vg is the specific volume of the saturated vapour and Vf is the specific volume of the saturated liquid.
28. At critical point, value of Vg-Vf is
a) two
b) one
c) zero
d) infinity
Answer: c
Explanation: As pressure increases, there is a decrease in Vg-Vf and at critical point its value becomes zero.
29. Above the critical point, the isotherms are continuous curves.
a) true
b) false
Answer: a
Explanation: These continuous curves approach equilateral hyperbolas at large volumes and low pressures.
30. A rigid tank contains 50 kg of saturated liquid water at 90°C. Determine the pressure in the tank and the volume of the tank.
a) 0.0518 m3
b) 0.0618 m3
c) 0.0718 m3
d) 0.0818 m3
Answer: a
Explanation: P = Psat@90 C = 70.183 kPa
v = vf@90 C = 0.001036 m3/kg
Total volume of the tank = mv = (50kg)( 0.001036 m3/kg)
= 0.0518 m3.
31. A piston –cylinder device contains 0.06m3 of saturated water vapour at 350 kPa pressure. Determine the temperature and mass of the vapour inside the cylinder.
a) 0.104 kg
b) 0.124 kg
c) 0.134 kg
d) 0.114 kg
Answer: d
Explanation: T = Tsat@350kPa = 138.86°C
v = vg@350kPa = 0.52422 m3/kg
m = V/v = 0.06 m3/0.52422 m3/kg = 0.114 kg.
32. Which of the following curves meet at triple point?
a) fusion curve and vaporization curve
b) fusion curve and sublimation curve
c) vaporization curve and sublimation curve
d) fusion curve and vaporization curve and sublimation curve
Answer: d
Explanation: At triple point, all these three curves meet.
33. The slopes of sublimation and vaporization curves for all substances are
a) negative
b) positive
c) zero
d) none of the mentioned
Answer: b
Explanation: This is true for all substances.
34. The slope of the fusion curve for water is
a) negative
b) positive
c) zero
d) none of the mentioned
Answer: a
Explanation: The slope of fusion curve for most substances is positive but for water it is negative.
35. The temperature at which a liquid boils is very sensitive to pressure but the temperature at which a solid melts is not such a strong function of pressure.
a) true
b) false
Answer: a
Explanation: The slope of the fusion curve is small.
36. Which of the following statement is true?
a) the triple point of water is 273.16 K
b) the triple point of CO2 is 216.55 K
c) when solid CO2 is exposed to 1atm pressure, it gets transformed into vapour directly
d) all of the mentioned
Answer: d
Explanation: The solid CO2 absorbs the latent heat of sublimation from the surroundings which gets cooled.
37. Quality indicates the
a) mass fraction of liquid in a liquid vapour mixture
b) mass fraction of vapour in a liquid vapour mixture
c) both of the mentioned
d) none of the mentioned
Answer: b
Explanation: Quality, x is given as mass of vapour divided by the total mass of liquid-vapour mixture.
39. If 1 kg of liquid-vapour mixture is considered and x is the quality of that mixture, then
a) mass of vapour is x kg
b) mass of liquid is (1-x) kg
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: Quality indicates the mass fraction of vapour in a liquid vapour mixture.
40. Which of the following statements is true?
a) the value of x varies between 0 and 1
b) for saturated water, x=0
c) for saturated vapour, x=1
d) all of the mentioned
Answer: d
Explanation: When water just starts boiling, x=0 and when vaporization is complete, x=1.
41. Total volume of a liquid vapour mixture is given by
a) volume of the saturated liquid
b) volume of the saturated vapour
c) sum of volumes of saturated liquid and saturated vapour
d) none of the mentioned
Answer: c
Explanation: V=Vf+Vg.
42. Voidage is given by
a) specific volume of saturated vapour / specific volume of liquid vapour mixture
b) specific volume of liquid vapour mixture / specific volume of saturated vapour
c) specific volume of saturated liquid / specific volume of liquid vapour mixture
d) specific volume of liquid vapour mixture / specific volume of saturated liquid
Answer: a
Explanation: Voidage is the volume fraction of vapour.
43. Specific volume of the mixture is given by
a) (1+x)vf + (x)vg
b) (1-x)vf + (x)vg
c) (1-x)vf – (x)vg
d) none of the mentioned
Answer: b
Explanation: Here vf=specific volume of saturated solid and vg=specific volume of saturated vapour.
44. Which of the following is correct?
a) v=vf + (x*vfg)
b) h=hf + (x*hfg)
c) s=sf + (x*sfg)
d) all of the mentioned
Answer: d
Explanation: Here, fg for each property is f-g for each property.
45. Which of the following statement is true about a chart of thermodynamic property?
a) the manner of variation of properties is clearly given in a chart
b) there is no problem in interpolation
c) the precision is not as much as in steam tables
d) all of the mentioned
Answer: d
Explanation: These are some advantages and disadvantages of a chart.
46. The temperature-entropy plot and enthalpy-entropy plot are commonly used.
a) true
b) false
Answer: a
Explanation: But its scale is small and limited in use.
47. Which of the following statement is true?
a) the temperature-entropy plot shows the vapour dome and the lines of constant volume, constant pressure, constant enthalpy, constant entropy and constant superheat
b) the scale of temperature-entropy plot is small which limits its use
c) enthalpy-entropy plot has a larger scale to provide data suitable for many computations
d) all of the mentioned
Answer: d
Explanation: These are some basic facts about temperature-entropy plot and enthalpy-entropy plot.
1. A gas compression process is
a) adiabatic
b) involves heat transfer
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: A gas compression process can be either adiabatic or can involve heat transfer.
2. If the gas is cooled during compression, work required will be ____ the adiabatic compression work.
a) more than
b) less than
c) equal to
d) none of the mentioned
Answer: b
Explanation: Here the work required will be less than that required for adiabatic compression.
3. Which of the following is an advantage of cooling?
a) less pipe friction losses
b) reduction in volume of gas
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: These are the two advantages of cooling.
4. We use after-coolers to cool the gas which leaves the compressor.
a) true
b) false
Answer: a
Explanation: This is done because compression process is somewhat ineffective.
5. The work of compression is ____ the shaft work.
a) positive of
b) negative of
c) equal to
d) less than
Answer: b
Explanation: This is true for reversible adiabatic compression.
6. For ɣ>n>1 and for same pressure ratio p2/p1, the maximum work is needed for
a) isothermal compression
b) adiabatic compression
c) polytropic compression
d) all need same work
Answer: b
Explanation: This comes when these three reversible compression processes are plotted on the p-V diagram.
7. In isothermal compression, all work done on gas is transformed into
a) heat added into system
b) heat going out of system
c) internal energy increase
d) none of the mentioned
Answer: c
Explanation: This is the case of isothermal compression.
8. When isothermal compression is taken as ideal process, the energy imparted
a) raises the temperature of gas
b) raises the pressure of gas
c) both of the mentioned
d) none of the mentioned
Answer: b
Explanation: In isothermal compression considered as ideal process, no energy is imparted to the gas.
9. The adiabatic efficiency is given by
a) Ws/Wc
b) Ws/Wt
c) Wt/Wc
d) Wt/Ws
Answer: a
Explanation: This is the efficiency of compressor working in a steady flow process.
10. The isothermal efficiency is given by
a) Ws/Wc
b) Ws/Wt
c) Wt/Wc
d) Wt/Ws
Answer: c
Explanation: This is the efficiency of compressor working in a steady flow process and Wt=work in reversible isothermal compression.
11. The adiabatic efficiency of real compressor can be ____
a) less than unity
b) greater than unity
c) equal to unity
d) none of the mentioned
Answer: b
Explanation: This is due to the effects of cooling.
12. For an adiabatic machine, work of compression is greater than enthalpy rise of gas.
a) true
b) false
Answer: b
Explanation: For an adiabatic machine, work of compression is equal to the enthalpy rise of gas.
13. Argon is kept in a 5 m3 tank at −30°C and 3 MPa. Determine the mass using compressibility factor.
a) 208.75 kg
b) 308.75 kg
c) 303.75 kg
d) 203.75 kg
Answer: b
Explanation: Tr = 243.15/150.8 = 1.612 and Pr = 3000/4870 = 0.616 hence Z = 0.96
m = PV/ZRT = (3000 × 5)/(0.96 × 0.2081 × 243.2)
= 308.75 kg.
14. Find the error in specific volume if ideal gas model is used to represent the behaviour of superheated ammonia at 40°C and 500 kPa?
a) 1.5%
b) 3.5%
c) 4.5%
d) 2.5%
Answer: c
Explanation: NH3, T = 40°C = 313.15 K, Tc = 405.5 K, Pc = 11.35 MPa
v = 0.2923 m3/kg
Ideal gas: v = RT/P = (0.48819 × 313)/(500) = 0.3056 m3/kg
thus error = 4.5%.
15. Find the volume of ethylene having mass of 125 kg at 7.5 MPa and 296.5 K.
a) 0.369 m3
b) 0.669 m3
c) 0.569 m3
d) 0.469 m3
Answer: d
Explanation: For ethylene, Tc = 282.4 K and Pc = 5.04 MPa
Tr = T/Tc = 296.5 / 282.4 = 1.05 and Pr = P/Pc = 7.5 / 5.04 = 1.49
thus Z = 0.32
hence V = mZRT / P = 125 × 0.32 × 0.2964 × 296.5 / 7500 = 0.469 m3.
16. The temperature and pressure conditions at free air delivery are
a) 27 degree Celsius, 100 bar
b) 15 degree Celsius, 101.325 bar
c) 27 degree Celsius, 101.325 bar
d) 15 degree Celsius, 100 bar
Answer: b
Explanation: This is known as FAD(free air delivery).
17. The volumetric efficiency is defined as the ratio of
a) total volume / piston displacement volume
b) total volume / gas volume taken during suction
c) gas volume taken during suction / swept volume
d) swept volume / gas volume taken during suction
Answer: c
Explanation: The swept volume is also called piston displacement volume.
18. Clearance is given by
a) total volume / swept volume
b) total volume / clearance volume
c) swept volume / clearance volume
d) clearance volume / swept volume
Answer: d
Explanation: Here C=Vc/Vs.
19. The clearance volumetric efficiency is equal to
a) 1 + C + C(p2/p1)^(1/n)
b) 1 – C – C(p2/p1)^(1/n)
c) 1 – C + C(p2/p1)^(1/n)
d) 1 + C – C(p2/p1)^(1/n)
20. As clearance and pressure ratio increases, volumetric efficiency ____
a) decreases
b) increases
c) remains constant
d) none of the mentioned
Answer: a
Explanation: This comes from the equation of volumetric efficiency.
21. To get maximum flow capacity, compressors are built with maximum practical clearance.
a) true
b) false
Answer: b
Explanation: To get maximum flow capacity, compressors are built with minimum practical clearance.
22. When the clearance volume is at minimum level,
a) volumetric efficiency is maximum
b) flow through machine is maximum
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: We can get this from volumetric efficiency equation.
23. For a given pressure ratio, volumetric efficiency is zero when maximum clearance is
a) 1 / ((p2/p1)^(1/n) +1)
b) 1 / ((p2/p1)^(1/n) -1)
c) 1 / ((p1/p2)^(1/n) -1)
d) 1 / ((p1/p2)^(1/n) +1)
Answer: b
Explanation: This comes from the equation of volumetric efficiency when we put volumetric efficiency equal to zero.
24. Increasing the pressure ratio, increases the volumetric efficiency.
a) true
b) false
Answer: b
Explanation: For a fixed clearance, increasing the pressure ratio decreases the volumetric efficiency.
25. Which of the following is correct?
a) p2max/p1 = (1 + 1/C)n
b) p2max/p1 = (1 – 1/C)n
c) p2max/p1 = (1 – C)n
d) p2max/p1 = (1 + C)n
Answer: a
Explanation: the maximum pressure ratio which can be attained by a reciprocating compressor cylinder is limited by the clearance.
26. For minimum work, the compression should be ____
a) adiabatic
b) isothermal
c) isochore
d) isobar
Answer: b
Explanation: This is the condition for minimum work.
27. The temperature after compression is given by
a) T2=(T1)(p2/p1)^(n/(n-1))
b) T2=(T1)(p2/p1)^((n+1)/n)
c) T2=(T1)(p2/p1)^((n-1)/n)
d) none of the mentioned
Answer: c
Explanation: Here T2 is the delivery temperature.
28. As the pressure ratio increases, delivery temperature ____
a) increases
b) decreases
c) remains constant
d) none of the mentioned
Answer: a
Explanation: This comes from the expression T2=(T1)(p2/p1)^((n-1)/n).
29. The volumetric efficiency ____ when carried out in stages.
a) decreases
b) increases
c) remains constant
d) none of the mentioned
Answer: b
Explanation: This is the reason why compression is carried out in stages.
30. The first stage of compression is done in ____ cylinder and next stage in ____ cylinder.
a) both in high pressure cylinder
b) both in low pressure cylinder
c) high pressure, low pressure
d) low pressure, high pressure
Answer: d
Explanation: This is how compression is carried out in two stages.
31. After passing through HP cylinder, the gas is passed to an intercooler.
a) true
b) false
Answer: b
Explanation: The gas is passed through an intercooler after it is compressed in LP cylinder.
32. In perfect intercooling, gas from intercooler has temperature equal to
a) inlet temperature
b) outlet temperature
c) intercooler temperature
d) all of the mentioned
Answer: a
Explanation: This is called complete or perfect intercooling when gas leaves the LP cylinder.
33. In perfect aftercooling, gas from intercooler has temperature equal to
a) inlet temperature
b) outlet temperature
c) intercooler temperature
d) all of the mentioned
Answer: b
Explanation: This is called complete or perfect aftercooling when gas leaves the HP cylinder.
34. The ideal intermediate pressure is given by
a) 2*p2/p1
b) sqrt(p2/p1)
c) (p1+p2)/2
d) sqrt(p1*p2)
Answer: d
Explanation: This is the ideal intermediate pressure for minimum work of compression.
35. The work required in HP compressor = work required in LP compressor.
a) true
b) false
Answer: a
Explanation: This happens when work of compression is minimum.
36. Rotary compressors are used where ____ quantities of gas are needed at relatively ____ pressure.
a) large, high
b) large, low
c) small, high
d) small, low
Answer: b
Explanation: This is where rotary compressors are used.
37. Rotary compressor can be classified as
a) displacement compressor
b) steady-flow compressor
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: These are the two types of rotary compressor.
38. In steady-flow compressor, compression occurs by
a) transfer of kinetic energy
b) transfer of potential energy
c) trapping air
d) all of the mentioned
Answer: a
Explanation: The transfer of kinetic energy occurs from a rotor.
39. In displacement compressor, compression occurs by
a) transfer of kinetic energy
b) transfer of potential energy
c) trapping air
d) all of the mentioned
Answer: c
Explanation: Here air is compressed by trapping it in reducing space.
40. The rotary positive displacement machines are ____ and compression is ____
a) cooled, isothermal
b) uncooled, isothermal
c) cooled, adiabatic
d) uncooled, adiabatic
Answer: d
Explanation: These are uncooled and adiabatic compression takes place.
41. The Roots blower and vane-type compressor are the types of
a) displacement compressor
b) steady-flow compressor
c) both of the mentioned
d) none of the mentioned
Answer: a
Explanation: These are the two types of rotary positive displacement machines.
42. For a Root blower, as pressure ratio increases, efficiency ____
a) increases
b) decreases
c) remains constant
d) none of the mentioned
Answer: b
Explanation: This can be seen by taking pressure ratios and calculating efficiencies for them.
43. The vane type compressor requires ____ the Roots blower.
a) equal work input
b) more work input
c) less work input
d) none of the mentioned
Answer: c
Explanation: This is true for given air flow and pressure ratio.
44. The centrifugal and axial flow compressor are the types of
a) displacement compressor
b) steady-flow compressor
c) both of the mentioned
d) none of the mentioned
Answer: b
Explanation: These are the two types of steady-flow compressors.
45. Which of the following is true for a centrifugal compressor?
a) rotation of impeller compresses the air
b) diffuser converts part of KE into internal energy
c) typical pressure ratio is around 1.4 to 1
d) all of the mentioned
Answer: d
Explanation: This is the working of a centrifugal compressor.
1. A power cycle continuously converts ____ into ____
a) heat, heat
b) work, heat
c) heat, work
d) work, work
Answer: c
Explanation: Here heat is the energy released by burning of fuel and work is done as shaft work.
2. In the vapour power cycle, working fluid undergoes a change of phase.
a) true
b) false
Answer: a
Explanation: Here working fluid is water.
3. The path followed in a vapour power cycle is
a) boiler-condenser-turbine-pump
b) boiler-turbine-condenser-pump
c) boiler-turbine-pump-condenser
d) boiler-pump-turbine-condenser
Answer: b
Explanation: In the boiler, water takes heat then expands in turbine going into condenser where it condenses into water and then it is pumped back into boiler.
4. For a fluid undergoing cycle process,
a) there is no net change in its internal energy
b) energy transfer as heat is equal to the energy transfer as work
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: When a fluid undergoes a cycle process, this changes take place.
5. For a vapour power cycle,
a) net heat input is converted into net work output
b) Q1-Q2 = Wt-Wp
c) efficiency = 1 – (Q2/Q1)
d) all of the mentioned
Answer: d
Explanation: Here Q1 is the heat transferred to the fluid and Q2 is the heat rejected, Wt is work transferred from fluid and Wp is work transferred into fluid.
6. In a Rankine cycle, all the processes are ideal.
a) true
b) false
Answer: a
Explanation: The Rankine cycle is an ideal cycle and also a reversible cycle.
7. For a Rankine cycle, which of the following is true?
a) a reversible constant pressure heating process happens in steam boiler
b) reversible adiabatic expansion of steam in turbine
c) reversible constant pressure heat rejection in condenser
d) all of the mentioned
Answer: d
Explanation: All the processes are ideal in Rankine cycle.
8. The liquid water handled by pump is
a) incompressible
b) with increase in pressure, there is a little change in density or specific volume
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: In a pump, reversible adiabatic compression of liquid takes place.
9. The work ratio is defined as the ratio of
a) positive work output to net work output
b) net work output to positive work output
c) heat input to work output
d) none of the mentioned
Answer: b
Explanation: The work ratio = Wnet / Wt.
10. Steam rate is the rate of steam flow required to produce unit shaft output.
a) true
b) false
Answer: a
Explanation: It is the capacity of a steam plant and steam rate = 1/(Wt-Wp).
11. Heat rate is given by (in kJ/kWh)
a) cycle efficiency
b) 3600 / cycle efficiency
c) cycle efficiency / 3600
d) cycle efficiency * 3600
Answer: b
Explanation: Heat rate is the rate input required to produce unit work output.
12. Which of the following statement is true?
a) during compression, specific volume of the fluid should be kept small
b) during expansion, specific volume of the fluid should be kept large
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: The larger the specific volume, more is the work produced or consumed by the steady-flow device.
13. Work output of turbine is ____ the work input to the pump.
a) much larger
b) much smaller
c) equal to
d) none of the mentioned
Answer: a
Explanation: This is the reason why steam power plants is so popular.
14. A power cycle continuously converts ____ into ____
a) heat, heat
b) work, heat
c) heat, work
d) work, work
Answer: c
Explanation: Here heat is the energy released by burning of fuel and work is done as shaft work.
15. In the vapour power cycle, working fluid undergoes a change of phase.
a) true
b) false
Answer: a
Explanation: Here working fluid is water.
16. The path followed in a vapour power cycle is
a) boiler-condenser-turbine-pump
b) boiler-turbine-condenser-pump
c) boiler-turbine-pump-condenser
d) boiler-pump-turbine-condenser
Answer: b
Explanation: In the boiler, water takes heat then expands in turbine going into condenser where it condenses into water and then it is pumped back into boiler.
17. For a fluid undergoing cycle process,
a) there is no net change in its internal energy
b) energy transfer as heat is equal to the energy transfer as work
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: When a fluid undergoes a cycle process, this changes take place.
18. For a vapour power cycle,
a) net heat input is converted into net work output
b) Q1-Q2 = Wt-Wp
c) efficiency = 1 – (Q2/Q1)
d) all of the mentioned
Answer: d
Explanation: Here Q1 is the heat transferred to the fluid and Q2 is the heat rejected, Wt is work transferred from fluid and Wp is work transferred into fluid.
19. In the Rankine cycle, heat is added reversibly at
a) constant pressure and constant temperature
b) constant pressure and infinite temperature
c) infinite pressure and constant temperature
d) infinite pressure and infinite temperature
Answer: b
Explanation: This is a basic fact about Rankine cycle.
20. The efficiency of Rankine cycle is given by
a) 1 – (Q1/Q2)
b) 1 – (Tmean/T2)
c) 1 – (T2/Tmean)
d) none of the mentioned
Answer: c
Explanation: Here T2 is the temperature of heat rejection and Tmean is the mean temperature of heat addition.
21. Which of the following statement is true?
a) for given Tmean, lower is the T2, higher will be the efficiency of Rankine cycle
b) the lowest possible temperature of heat rejection is the surroundings temperature
c) higher is the mean temperature of heat addition, higher will be the efficiency
d) all of the mentioned
Answer: d
Explanation: The efficiency of the Rankine cycle = 1 – (T2/Tmean).
22. If we ____ the superheat at constant pressure then the cycle efficiency ____
a) decrease, increases
b) increase, decreases
c) increase, increases
d) decrease, decreases
Answer: c
Explanation: Increasing the superheat at constant pressure increases the mean temperature of heat addition and cycle efficiency also increases.
23. The maximum temperature of steam that can be used is not fixed.
a) true
b) false
Answer: b
Explanation: It is fixed from metallurgical considerations.
24. The mean temperature of heat addition can be increased by
a) increasing the amount of heat supplied at high temperatures
b) decreasing the amount of heat added at low temperatures
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: These are the two ways of increasing mean temperature of heat addition.
25. In the ideal regenerative cycle, the condensate after leaving the pump circulates around the turbine casing.
a) true
b) false
Answer: a
Explanation: Through this heat transfer takes place between the vapour flowing through the turbine and liquid flowing around the turbine.
26. The efficiency of an ideal regenerative cycle is given by
a) 1 – (T1/T2)
b) 1 – (T2/T1)
c) 1 – (Q1/Q2)
d) none of the mentioned
Answer: b
Explanation: The efficiency of a cycle is given by 1 – (Q2/Q1).
27. The efficiency of an ideal regenerative cycle is ____ the Carnot cycle efficiency.
a) greater than
b) equal to
c) less than
d) none of the mentioned
Answer: b
Explanation: For both the cycles, efficiency is given by 1 – (T2/T1).
28. When compared with the Rankine cycle, the ideal regenerative cycle has
a) less net work output
b) more steam rate
c) more efficient
d) all of the mentioned
Answer: d
Explanation: These indiate that the ideal regenerative cycle is better than the Rankine cycle but it is not practicable.
29. The Otto cycle is the
a) air standard cycle of CI engine
b) air standard cycle of SI engine
c) vapour power cycle of CI engine
d) vapour power cycle of SI engine
Answer: b
Explanation: The Otto cycle is air standard cycle and is used in SI engine.
30. In a four-stroke internal combustion engine,
a) the piston does four complete strokes within cylinder
b) for each cycle, the crankshaft completes two revolutions
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: This is the functioning of a four-stroke internal combustion engine.
31. The correct sequence of strokes in a four-stroke SI engine is
a) intake->compression->exhaust->expansion
b) intake->expansion->compression->exhaust
c) intake->exhaust->compression->expansion
d) intake->compression->expansion->exhaust
Answer: d
Explanation: The correct sequence is intake->compression->expansion->exhaust and expansion stroke is also called power stroke.
32. The spark plug fires shortly before the ____ stroke.
a) compression
b) expansion
c) intake
d) exhaust
Answer: b
Explanation: The spark plug fires shortly before the piston reaches TDC and after this ignition the expansion stroke takes place.
33. The pressure in cylinder is ____ the atmospheric value during exhaust stroke and ____ it during intake stroke.
a) above, below
b) below, above
c) equal to, equal to
d) equal to, above
Answer: a
Explanation: This is done to ensure that all the exhaust gases are thrown out of the cylinder and enough amount of intake mixture enters the cylinder.
34. In SI engines,
a) air-fuel mixture is compressed
b) compression ratio is limited
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: The onset of engine knock or auto-ignition limits the compression ratio in SI engines.
35. In CI engines,
a) during compression stroke, only air is compressed
b) compression ratios can be much higher
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: This is an advantage of CI engine over SI engine.
36. The correct sequence of processes in CI engine is
a) intake->fuel injection and combustion->compression->expansion->exhaust
b) intake->compression->fuel injection and combustion->expansion->exhaust
c) intake->compression->expansion->fuel injection and combustion->exhaust
d) intake->compression->exhaust->fuel injection and combustion->expansion
Answer: b
Explanation: The correct sequence of processes in CI engine is intake->compression->fuel injection and combustion->expansion->exhaust.
37. The processes in CI engine cycle is completed in ____ strokes of piston and ____ revolutions of crankshaft.
a) four, four
b) two, two
c) two, four
d) four, two
Answer: d
Explanation: There are four strokes and number of revolutions of crankshaft required are two.
38. The Diesel cycle consists of
a) two reversible isotherms and two reversible isobars
b) one reversible isochore and two reversible adiabatics and one reversible isobar
c) one reversible isotherm and two reversible isochores and one reversible isobar
d) two reversible isobars and two reversible adiabatics
Answer: b
Explanation: These four processes comprises Diesel cycle.
39. A gas turbine power plant uses
a) Otto cycle
b) Rankine cycle
c) Brayton cycle
d) Diesel cycle
Answer: c
Explanation: The Brayton cycle is the air standard cycle for gas turbine power plant.
40. The Brayton cycle consists of
a) two reversible isotherms and two reversible isobars
b) two reversible isochores and two reversible adiabatics
c) two reversible isotherms and two reversible isochores
d) two reversible isobars and two reversible adiabatics
Answer: d
Explanation: These are the processes of Brayton cycle.
41. Which of the following is true for the Brayton cycle?
a) first sir is compressed reversibly and adiabatically
b) heat is added reversibly at constant pressure
c) air expands in turbine reversibly and adiabatically
d) all of the mentioned
Answer: d
Explanation: These processes take place in the Brayton cycle.
42. The efficiency of Brayton cycle is given by (rk is the compression ratio)
a) 1/(rk)^(ɣ-1)
b) 1 – 1/(rk)^(ɣ)
c) 1 – 1/(rk)^(ɣ-1)
d) 1/(rk)^(ɣ)
Answer: c
Explanation: This is the expression for efficiency of Brayton cycle and rk=compression ratio.
43. The efficiency of Brayton cycle depends on
a) compression ratio
b) pressure ratio
c) either compression ratio or pressure ratio
d) both compression ratio and pressure ratio
Answer: c
Explanation: The reason being, compression ratio can be expressed in terms of pressure ratio.
44. For the same compression ratio, the efficiency of Brayton cycle is ____ the efficiency of Otto cycle.
a) less than
b) equal to
c) greater than
d) none of the mentioned
Answer: b
Explanation: The expressions for efficiency of Brayton cycle and Otto cycle are same.
45. Both Rankine cycle and Brayton cycle consists of two reversible isochores and two reversible adiabatics.
a) true
b) false
Answer: b
Explanation: Both Rankine cycle and Brayton cycle consists of two reversible isobars and two reversible adiabatics.
46. In Rankine cycle, working fluid ____ , in Brayton cycle working fluid ____
a) undergoes phase change, remains in gaseous phase
b) remains in gaseous phase, undergoes phase change
c) undergoes phase change, undergoes phase change
d) remains in gaseous phase, remains in gaseous phase
Answer: a
Explanation: This is a difference in Rankine cycle and Brayton cycle.
47. For Brayton cycle, average specific volume of air that compressor handles is ____ the same of gas in a gas turbine.
a) equal to
b) more than
c) less than
d) none of the mentioned
Answer: c
Explanation: This is because the gas temperature is much higher.
48. A steam power plant works on ____ and a gas turbine works on ____
a) both work on Rankine cycle
b) both work on Brayton cycle
c) Brayton cycle, Rankine cycle
d) Rankine cycle, Brayton cycle
Answer: d
Explanation: This is a difference in a steam power plant and a gas turbine.
Prepare For Your Placements: https://lastmomenttuitions.com/courses/placement-preparation/
![]()
/ Youtube Channel: https://www.youtube.com/channel/UCGFNZxMqKLsqWERX_N2f08Q
Follow For Latest Updates, Study Tips & More Content!
Not a member yet? Register now
Are you a member? Login now